MAGNETO-STRUCTURAL CORRELATIONS IN LOW-DIMENSIONAL MAGNETS FORMED BY NON-COVALENT INTERACTIONS
A. Orendáčová, E. Čižmár, M. Orendáč, M. Kajňaková, J. Černák, A. Feher.
Over the past few years, non-covalent interactions, namely hydrogen bonds (HBs) have attracted a considerable amount of attention due to their important role in biological systems as well as in structural chemistry. Their flexibility and variability enables to organize the assembly of building blocks into stabile three-dimensional crystal structures. Recent quantum mechanical calculations of exchange coupling in Cu(II) dimers formed by HBs of O-H···O sort revealed that the HBs play predominantly structural role, e.i. they are responsible for the shortening of the distance between atoms in the absence of covalent bonding. The shortening enables the formation of unconventional exchange pathways [1]. This property together with the flexibility and variability predetermines HBs as a promising new way for creation of novel quantum magnetic systems.
Recent theoretical studies have shown that the Néel order, present in the ground state of the quantum S = 1/2 isotropic square lattice, can "melt" due to the effect of spatial anisotropy and/or next-nearest-neighbour magnetic correlations. The latter introduces geometrical frustration, thus such low-dimensional magnets are attractive objects for the investigation of the interplay of quantum fluctuations and frustration effects. The realization of such objects can be rather easily achieved via the HBs.
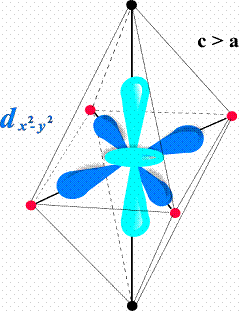
Fig.1
Cu(II) cyanocomplexes characterized by a chain-like crystal structure proved to represent spatially anisotropic two-dimensional (2d) magnets. The local surroundings of Cu(II) ion formed by a ligand octahedron elongated due to Jahn-Teller distortion, stabilizes dz2 electronic ground state of the Cu(II) ion. It implicates a preferred formation of exchange pathways within the equatorial plane of the local octahedron along dx2-y2 lobes (Fig.1), perpendicular to the covalent chains. Since the chain-like structure does not offer any covalent bonds between the chains, the main role in the formation of exchange pathways is played be the present HBs. In other words, the chain-like crystal structure does not coincide with the magnetic structure which is built in the layers perpendicular to the covalent chains. The symmetry of the 2d lattice is determined by the position of Cu(II) ions and HBs geometry and can vary from square to triangular pattern. Our systematic investigations of magnetic properties of cyanocomplexes are summarized in the Ref. 2.
Cu(en)2Ni(CN)4 with chain-like crystal structure is a good representative of the Heisenberg square lattice with the intralayer coupling J/kB = -0.36 K formed due to the presence of hydrogen bonds in the equatorial plane of local ocathedron [3]. The appearance of a phase transition to the ordered state at Tc = 0.13 K in this compound was interpreted as a combined effect of a potential exchange Ising-like anisotropy and interlayer magnetic coupling which drives the transition to the 3d Ising universality class. This conjecture was confirmed later by detailed theoretical studies of the Heisenberg square lattice with spin 1/2, focused at the influence of extremely weak exchange anisotropy l = 1.001 - 1.01 [4]. The studies found that the critical temperatures stay finite for any finite exchange anisotropy and, strong quantum fluctuations characteristic for low-dimensional quantum systems cannot destroy the transition. A weak Ising-like exchange anisotropy can be expected in Cu(en)2Ni(CN)4 due to the g-factor anisotropy of Ising type, yielding typical values l » 1.01.
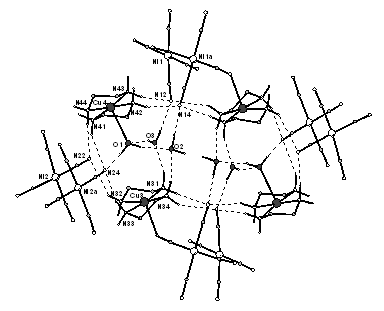
A cyanocomplex compound [CuII(en)2(H2O)] [CuII(en)2Ni2Cu2I(CN)10].2H2O deserves a spatial attention as an example of a molecular magnet in which copper paramagnetic centers are connected into centro symmetric tetrameric units via the HBs [5]. Corresponding magnetic subsystem can be approximated by a model of a planar tetramer with three different couplings J1, J2 and J3 » 1K (Fig.2). However, specific heat data clearly indicate the need to incorporate further interactions introduced by HBs.
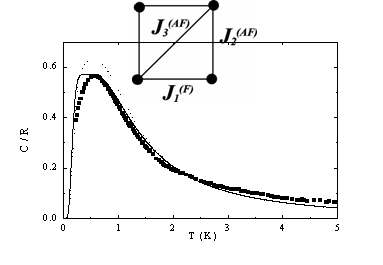
Fig.2
[1] C. Desplanches, E. Ruiz, A. Rodríguez-Fortea, S. Alvarez, J. Am. Chem. Soc. 124 (2002)
5197.
[2] J. Černák, M. Orendáč, I. Potočňák, J. Chomič, A. Orendáčová, J. Skoršepa, A. Feher,
Coordination Chemistry Review 224 (2001) 51.
[3] M. Orendáč, A. Orendáčová, J. Černák, A. Feher, Solid State Communications 94 (1995) 833.
[4] A. Cuccoli, T. Roscilde, V. Tognetti, R. Vaia, P. Verruchi, cond - mat/0209316.
[5] A. Orendáčová, M. Kajňaková, J. Černák, J. –H. Park, E. Čižmár, M. Orendáč, A. Vlček,
O.V. Kravchyna, A.G. Anders, A. Feher, M.W. Meisel, Chem. Phys. 309 (2005) 115.