Magnets of lower dimensions containing non-covalent bonds
Current experimental studies of geometrically frustrated magnets and other types of low dimensional magnets ultimately require materials with specific spatial arrangement of exchange paths. Although several strategies have been developed to synthesize these materials, yet the avenues to the synthesis of a material with the desired topology of exchange paths is not straightforward. Consequently, different approaches are searched relying on materials from various classes, specifically those which, apart from conventional covalent bonds, contain other types of bonds.
Hydrogen bond belongs to an example of such unconventional bond. Although its energy may be several orders of magnitude smaller than that of a covalent bond, it plays a crucial role not only in solid state physics or chemistry, but also in biological objects. Combining both covalent and hydrogen bonds in a material leads to variable networks of bonding schemes in which different exchange interactions can be mediated. Consequently, the aforementioned approach may be successfully adopted for investigating magnetic systems in which spatial anisotropy of exchange interaction is desirable. So-called Row model for geometrically frustrated magnet may serve as an example [1]. The model assumes spins located in triangular lattice with different values of exchange interaction mediated in various directions, see Fig. 1.
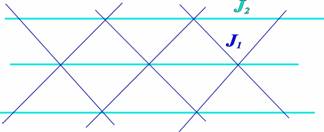
Fig. 1 Schematic plot of a Row model on a triangular lattice.
Magnetic properties of the model strongly depend on the ratio of exchange couplings ?=J2/( J1+J2). Specifically, a Néel type ordered phase exists from ?=0 to ?=1/3, a phase with spiral ground state is located in the region 1/3 < ? < 0.8 and quantum disordered phases are predicted at ?=1/3 and ??0.8 [2, 3], see Fig. 2.
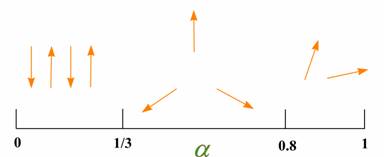
Fig. 2 Phase diagram proposed for Row model.
Our subsequent experimental studies of selected materials containing hydrogen bonds confirmed their ability in mediating exchange interaction of order of Kelvin. The hydrogen bonds significantly influence magnetic properties of the studied compounds. More specifically, in Cu(en)(H2O)2SO4 (en = ethylendiamine) the analysis of thermodynamic quantities and electron-spin resonance spectra revealed that despite the triangular arrangement of chemical bonds, the studied material is identified as an S=1/2 Heisenberg antiferromagnet where the dominant exchange coupling J/k=-1.4 K is mediated by a square network of hydrogen bonds [4]. The long-range ordering observed at 0.91 K was proposed to be of a Néel type. Systematic studies of compounds derived from Cu(en)(H2O)2SO4 in which en was replaced by e.g. imid, nad (imid = imidazole, nad = nicotinamide) revealed pronounced changes in both structure and magnetic properties. Both Cu(imid)(H2O)2SO4 and Cu(nad)(H2O)2SO4 were identified as S=1/2 zigzag ladders with exchange interactions much weaker than found in Cu(en)(H2O)2SO4 [5]. Future effort will be concentrated on materials from different classes in which higher values of exchange coupling may be expected.
References:
[1] U. Bhaumik et al., Phys. Rev. B 58 (1998) 73.
[2] A. E. Trumper et al., Phys. Rev. B 60 (1999) 2987.
[3] Z. Weihong et al., Phys. Rev. B 59 (1999) 14 367.
[4] M. Kajòaková et al., Phys. Rev. B 71 (2005) 014435.
[5] M. Kajòaková et al.,Czech. J. Phys.,54 (2004) D563.